The protein Rad52 is already known for its role in DNA repair. However, it may also be a choice target for treatments against a number of breast and ovarian cancers. Indeed, inhibiting Rad52 appears to lead to the death of tumor cells while causing no harm to healthy cells. Nonetheless, to truly grasp the function of Rad52, more fundamental data must be collected. Thus, an IRCM team, specialized in DNA repair mechanisms, decided to take a close look at that protein.
DNA repair mechanisms are essential to cell survival. One of them, homologous recombination (HR), plays a central role in maintaining genome stability. HR is very highly conserved; it is present in all living organisms, from bacteria to humans. A recombinase, Rad51, is vital in HR. It forms filaments on damaged DNA that identify homologous DNA sequences for subsequent use as a template to ensure an exact repair of the lesion. Eric Coïc's team at IRCM's Laboratory of Genetic and Molecular Radiobiology (LRGM) studies the mechanisms that closely control and regulate the proper formation of these Rad51 filaments and the search for homology. The team works with Saccharomyces cerevisiae, more commonly known as baker's yeast, a model organism very frequently used for minutiose study of many biological mechanisms. HR in baker's yeast is better understood today, with a number of positive and negative regulators having been identified. "In baker's yeast, the Rad52 protein was thought to be essential for the recruitment of Rad51 at lesions, and what happened to it after filament formation had remained a mystery", explains Eric Coïc. "However, our site-directed mutagenesis research has shown that interaction between Rad52 and Rad51 is not necessary for the formation of Rad51 filaments. But particularly, we've also shown that Rad52 remains associated with the Rad51 filaments and participates in their stabilization and protection against the displacement effect of another protein, Srs2", he adds. In humans, Rad52 is conserved but the recruitment of Rad51 at lesions is mainly assured by Brca2, a protein coded by the tumor suppressor gene BRCA2. A difference between us and yeast thus, but a question arises: Could Rad52 nonetheless play a role in the formation and the stabilization of Rad51 filaments in humans? The LRGM team's work suggests that perhaps it does, but in a particular and as of yet undefined context.
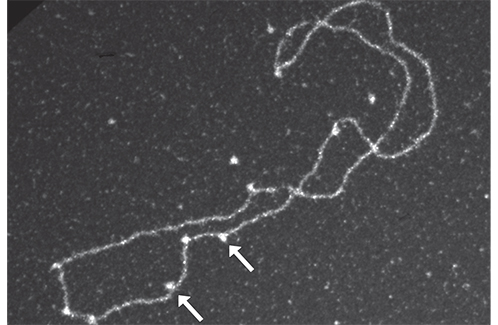
Electron microscopy observation: the arrows indicate Rad52-Rad51 complexes on a DNA filament.
© ma and al. eLife / IRCM/CEA