These complementary and totally integrated platforms can be used to carry out research projects in environmental biology requiring level 1, 2 or 3 biological confinement. Support teams consisting of experienced researchers, research officers and technicians are responsible for the daily running of these facilities.
The know-how of its support teams and the CRC MIRCen platforms themselves are available to academic and industrial research projects (see "service request“) to which the methodologies developed at MIRCen can be applied.
Other projects on infectious, hepatic or cardiac diseases can also be considered. Running such projects may require the development of complementary expertise and know-how, particularly in terms of MRI and PET methodology.
The MIRCen platforms have facilities for housing animals for these projects. The zootechnical team of the platforms is responsible for the housing and care of the animals. In particular, it ensures that they are correctly socialized and implements the enrichment programs necessary for their well-being. Also, the veterinary surgeon of the CRC MIRCen is responsible for the care of diseased animals. The veterinary surgeon, together with the
local ethics committee, is responsible for ensuring the ethical monitoring of projects. In accordance with decree 2013-118 of European directive 2010/63/UE, all projects are submitted to an institutional review board for approval. This board may, if necessary, refuse to give approval or may demand modifications to improve the treatment and conditions of the animals used for experimentation.
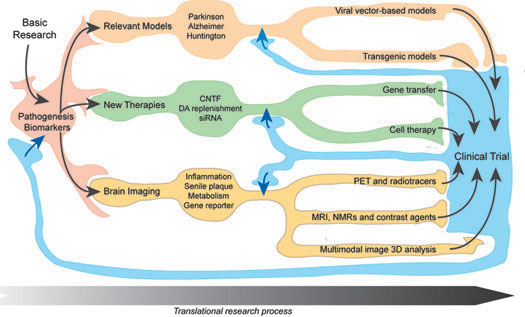
The MIRCen platforms are organized around a translational axis extending from the in vitro platforms to the analysis of data after the evaluation of new treatments or animal models.